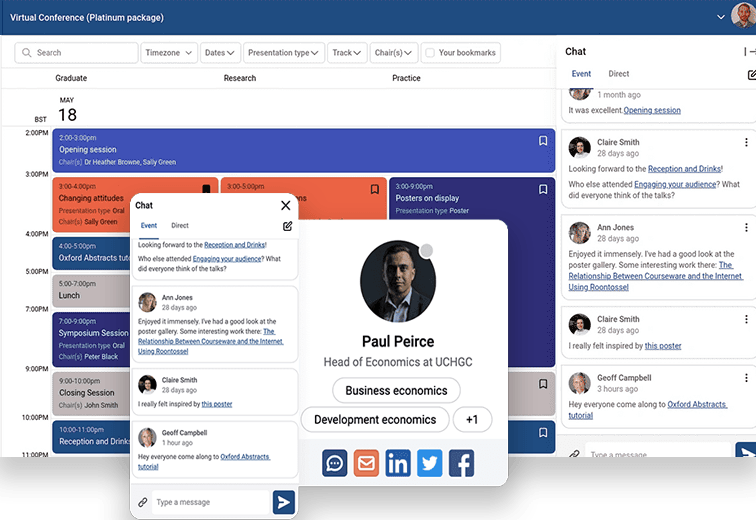
Abstract Management
Make your next call for abstracts the easiest yet.
Simplify even the most complex abstract management process
Customise submission, review and decision forms to collect the info you need
Extensive report options, and custom abstract books all included





Collect. Review. Decide.
Easy to set up, even easier to manage.
Unlimited submissions
- From just a handful, to thousands of submissions, our plans scale with your event, at no extra cost.
Custom forms
- Create and publish submission, review and decision forms from a huge range of templates.
Peer review
- Keep your reviewers happy with a user-friendly, feature-rich platform.
Manage
- Stay on track with a multi-view table, to access the data you need.
Reports
- Create standard and custom reports throughout the entire event process.
Publishing
- Download standard and custom abstract books and reports in real time.
Symposium
- Flexible options for grouping abstracts - for panels, workshops and roundtables.
Multi-stage
- Keep control of multi-stage processes with workflow tools and user-friendly interfaces.
1. Collect
We provide a simple, user friendly way to collect and manage your abstract and paper submissions.
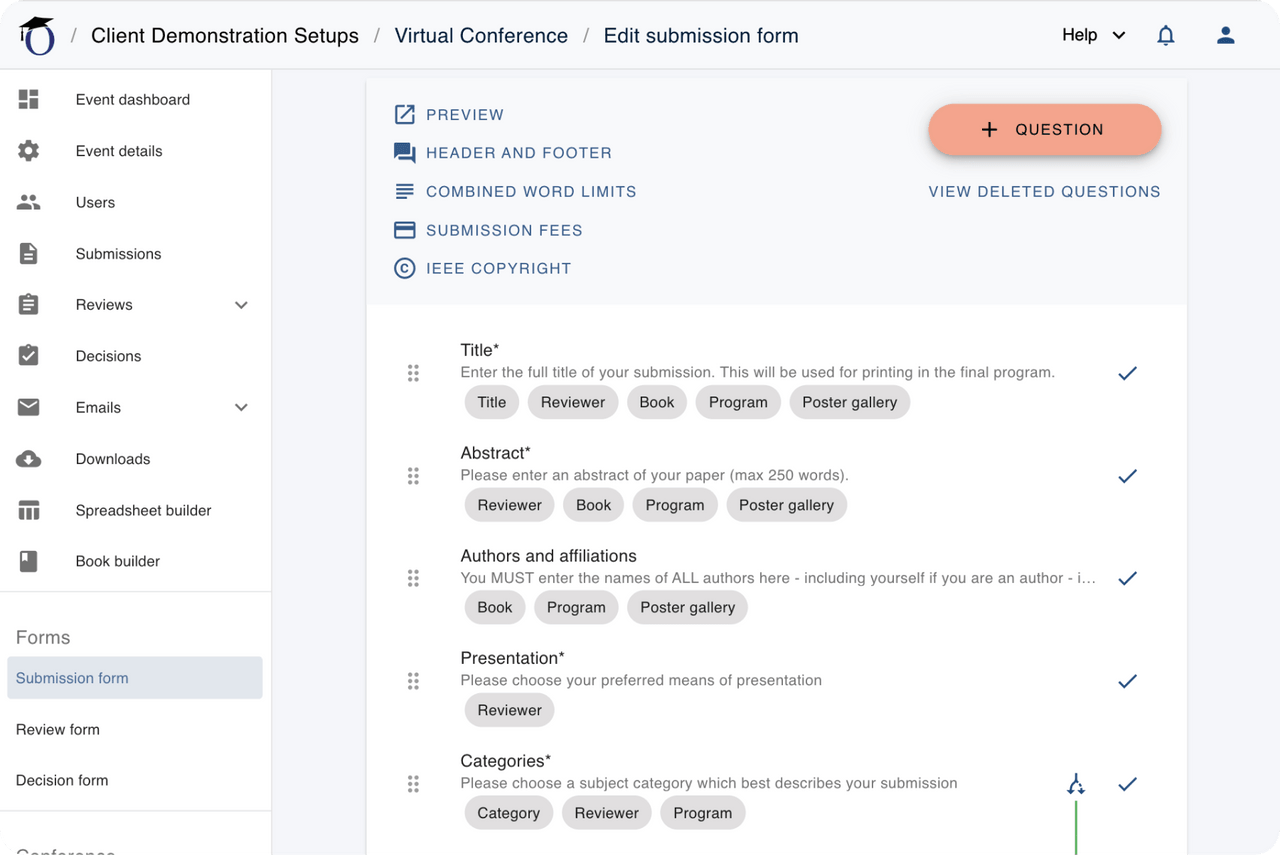
Create submission forms
- Choose from a comprehensive range of fully-editable key question templates, to collect exactly the data and information you need.
Open the call for papers
- Publish in a click, share and promote on your website, in emails and on social media, with embeddable links.
Collect and manage
- View, delete, withdraw, edit and download submissions and data, with a multi-view table and a range of user-friendly, intuitive tools.
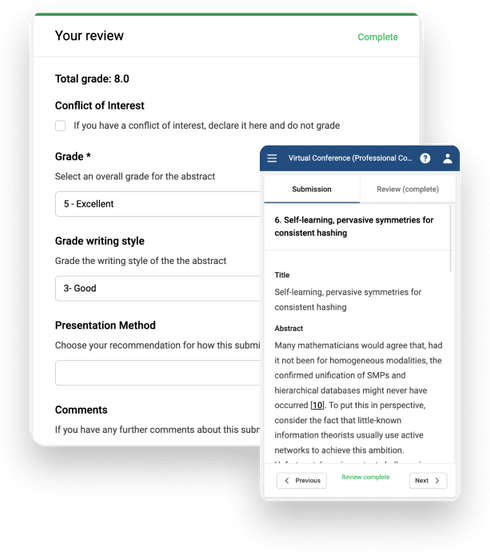
2. Review
We've created a system that is simple to use, but packed with features to help reviewers, and admins, to get the data they need.
Add and assign reviewers
- Add reviewers with just their email address, then assign abstracts and papers, individually, by specialism, and in bulk.
Collect reviews
- Keep reviewers engaged with a simple log in, a user-friendly interface, and submissions and grading form side-by-side on the same screen.
Manage reviewers
- Keep on top of late and incomplete reviews by tracking progress, and an integrated chase-up process.
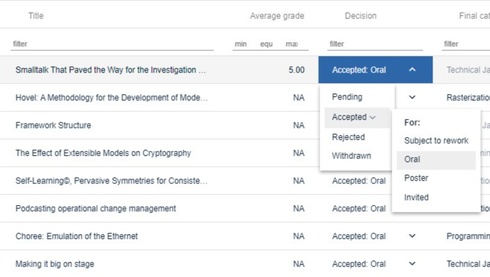
3. Decide
Simplify decision making on submissions with our powerful tools and streamlined process.
Create forms
- Design decision forms from a range of questions, create acceptance types and control permissions and visibility for your committee.
Make decisions
- Access all the data you need to accept, reject, mark for rework, (or any other outcome you create). Notify authors with integrated automatic and manual email tool.
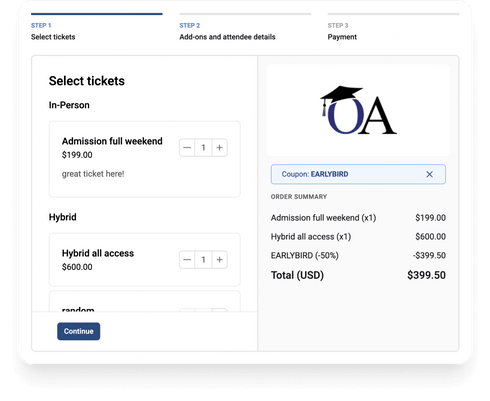
4. Sell Tickets
Create and sell tickets seamlessly using the Oxford Abstracts event registration.
Create your tickets
- Build flexible forms, multiple ticket types and even discount codes if required.
Set up currency & payment options
- Take payments easily via Stripe, Paypal & Authorize.net
Manage & Track Payments
- Get a complete overview of ticket sales in the dashboard. Send friendly payment reminders.
5. Report and Publish
From a range of instantly generated pdf downloads, to fully customised abstract books and books of proceedings, we offer peerless research conference reporting and publishing.
Communicate and notify
- Communicate with submitters, reviewers and committee members with customisable manual and automatic email templates
Create your abstract book
- Export any data, and download standard and custom abstract books and reports in real time.
Reports
- Choose from a standard range of popular reports, or create your own with the data you need.
Compare our packages
We offer a range of packages for all research conference requirements, from simple abstract management to a full virtual conference platform.
We believe in transparent pricing, so the price you see if the price you pay. Simply choose the package to suit you.
To see a full list of the features in each package, check out our Pricing page.
FREE
For basic abstract management
Features
Unlimited submissions
Collect, review, decide
Delegate Registration
Custom branding
Custom review and decision forms
Custom emails and abstract books
Abstract management
For more complex abstract management
Features
Unlimited submissions
Custom forms
Delegate Registration
Custom branding
Custom emails and abstract books
Advanced admin features
Extras, if you need them
For more complex abstract management, just select one of our add-ons, which integrate seamlessly with all our paid packages.
We've got your next call for abstracts covered
Take a look at our abstract management software in action.

“I have been very excited to have finally found abstract management software that is a great fit for our medical meeting. Even though we are a small meeting, the customer service and technical support has been excellent. We are using this for the first time and our review committee of 10 physicians have all found the process to be well organized and very easy.”

“I just think it is great, easy to use, does everything I need, great support if needed and fantastic value for money”

“Both the abstract submission process and the reviewing committee area are user-friendly and very efficient. It is an excellent system and very good value. Highly recommended by our scientific committee and our office staff who love the friendliness, efficiency and speed of the support from OA (not that it's needed). ”
Looking for a complete conference platform?
Run your entire conference with Oxford Abstracts
If you need more than just abstract management, take a look at our full conference platform with features such as program creator, delegate networking, delegate registration and much more!
Learn More →